Formulating for health
Reformulation used to be all about a product looking and tasting good and having a very long shelf life. These days it's very different, with consumers becoming more vocal in asserting which ingredients they want to see more of or less of in products.
Reformulation used to be so easy. It was all about the product; it had to look and taste good, and have a very long shelf life. The manner by which this was achieved was less important, with combinations of ingredients, processing methodologies and packaging resulting in high quality products. These days the situation is very different. The products are still important but so are the ingredients as consumers become more vocal in asserting which ingredients they want to see more or less of in products.
Reformulation challenges
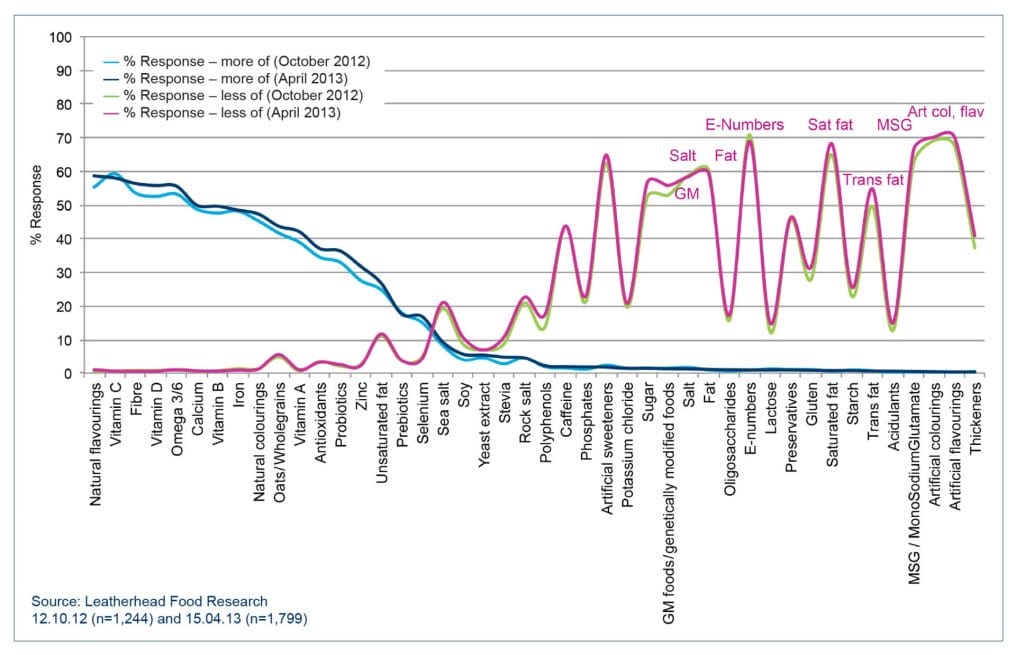
Consumers may not read the back-of-pack cooking instructions, but they certainly know which ingredients they want more and less of. Leatherhead Food Research regularly conducts consumer surveys on this subject (see Figure 1) and the ingredients that consumers would like more of are those that may be considered to be 'healthy', such as fibre, vitamin C, and omega 3/6 oils. Unsurprisingly the ingredients that consumers would like to see less of are those that may be considered to be 'unhealthy' such as saturated fat, salt and sugar.
| Figure 1 – Ingredients consumers want to see more of and less of in food and drink products. Click here or on the image above to see a larger version of the chart. For further information about this research, please contact Lucy Button, Business Development Executive, Sensory, Consumer & Market Insight. |
The challenge therefore in formulating foods for health is to increase the 'more desired' ingredients and reduce the 'less desired' ones, whilst maintaining delivery on a number of key criteria as follows:
- Food safety and stability
- Taste, texture and appearance
- Label cleanliness
- Shelf-life stability
- Functionality in use
- Ease of manufacturing
- Cost in use
Salt, fat and sugar reduction
Salt, fat and sugar, and in particular saturated and trans fats, are examples of the ingredients that consumers would like to see less of in their foods. Trans fats have largely been formulated out of foods, and the levels of saturated fats have decreased thanks to processing and purification technologies that have resulted in enhanced structuring capabilities with 'softer' oils.
Interesterification is an example of such a processing technology. Of course, reducing the total fat level in a food will also reduce both the saturates and trans levels, and formulators have a plethora of fat replacement ingredients and mimetics to choose from. Likewise there are ranges of salt replacement and sugar reduction ingredients available. The problem however in all three cases is that these ingredients don’t always taste like the real thing. For example, the most popular salt replacer, potassium chloride, can have a bitter taste at higher concentrations, and stevia for sugar reduction can have liquorice notes and a bitter aftertaste.
The best solutions therefore for salt, fat and sugar reduction are likely to be those that rely on enhancing the functionality of the ingredient itself such that the performance in the food material can be maintained at lower levels. A great example of this is the use of particle size. Reducing the size of sugar or salt crystals can result in enhanced dissolution in the mouth and high perceived sweetness or saltiness. Fats can be cryo-crystallised using liquid nitrogen to result in micron-sized particles rather than a continuous network, and then applied in bakery products to reduce the total and saturated fat levels.
Emulsion technology has a lot to offer in this space. Most foods contain oil and water, which means that some degree of emulsification is required to form oil-in-water (O/W) systems such as sauces and dressings, or water-in-oil (W/O) systems such as spreads and pastes. The use of double emulsion technology, in which the oil phase of an O/W emulsion has been replaced by a W/O emulsion to result in a water-in-oil-in-water (W/O/W) system, has potential for reducing salt, fat and sugar. A light micrograph of a W/O/W emulsion is shown in Figure 2. The effectiveness of such a system depends on the structure being maintained until at least the point of swallowing.
In fat reduction, the bulking out of the oil phase with internal water has been shown to be effective in maintaining the physical and organoleptic properties of dressings and mayonnaise-type products. In the case of salt reduction, preparing a W/O/W emulsion with all of the salt in the external water phase has been shown to enhance saltiness compared to an O/W emulsion with the same total salt level. With this approach also likely to be successful for sugar reduction, perhaps W/O/W emulsion technology is the silver bullet for reducing the levels of ‘less desired’ ingredients in foods!
Health claims
Health claims are currently a hot EU food law topic. The first piece of EU legislation laying down specific rules governing the use of nutrition and health claims made on foods was introduced in 2006 by Regulation (EC) No. 1924/2006. Whilst the closed list of nutrition claims has been included under the Annex, separate pieces of legislation were required authorising the use of health claims.
Over the past five-and-a-half years, since the EU legislation on nutrition and health claims was published, there has been a revolution in the way food labels are presented. The controversial Regulation (EU) No. 432/2012 establishing the list of general function health claims (Article 13.1 claims) was published in May 2012 and applied from 14 December 2012. The Regulation provides more clarity for the food industry in terms of which claims can and which cannot be used on foodstuffs. Looking into the numbers of approved claims (just over 220) in comparison to rejected ones (more than 2000), the marketing strategy likely needs to be changed for several products. The rejected claims are no longer permitted for use on foodstuffs. The Regulation does not explicitly provide longer transitional periods for foodstuffs placed on the market before the application date. In the UK, enforcement authorities, via the Association of Chief Trading Standards Officers, have recommended a pragmatic approach.
Some flexibility of wording of the approved health claims is possible provided its aim is to help consumer understanding, taking into account factors such as linguistic and cultural variations and the target population. Adapted wording must have the same meaning for the consumer as the authorised claim.
The elephant in the room remains claims currently 'on hold' (such as botanical health claims) while the European Commission has the difficult decision of what exactly to do with them. This means that many of these claims currently sit in a transitional period with no defined endpoint. The EU regulation was intended to include botanical health claims, though it became apparent that there was overlap with an existing framework for traditional herbal medicines. This presents both regulators and food marketers with a problem.
Now that the 222 harmonised and scientifically substantiated Article 13.1 health claims have been published, it has become even more important for the food industry to focus on establishing new scientific evidence for future health claim applications under Article 13.5 or Article 14 (approved separately under individual Regulations).
These are challenging times for food regulators and marketers and no doubt the regulatory landscape will be influenced by enforcement and future decisions on health claims. For assistance in navigating this difficult area of regulation please contact Leatherhead's Regulatory team.
Healthy ingredients
The combination of regulatory approval and consumer acceptance is a powerful motivation for food companies to invest in any ingredient or food. Reformulating foods for improved health credentials may therefore be concerned with adding or increasing the levels of such ingredients. This may either be instead of or in combination with reducing the levels of less desired or ‘unhealthy’ ingredients.
Vitamin C is an example of an ingredient that consumers would like to see more of in their foods and as such it can be safely assumed to be healthy. Indeed the long association with orange juice in particular has fixed this in consumers' minds and it is often supplemented in foods whether they carry health-claim messaging or not. It also has no less than 15 approved health claims as follows:
- Contributes to maintain the normal function of the immune system during and after intense physical exercise
- Contributes to normal collagen formation for the normal function of blood vessels
- Contributes to normal collagen formation for the normal function of bones
- Contributes to normal collagen formation for the normal function of cartilage
- Contributes to normal collagen formation for the normal function of gums
- Contributes to normal collagen formation for the normal function of skin
- Contributes to normal collagen formation for the normal function of teeth
- Contributes to normal energy-yielding metabolism
- Contributes to normal functioning of the nervous system
- Contributes to normal psychological function
- Contributes to the normal function of the immune system
- Contributes to the protection of cells from oxidative stress
- Contributes to the reduction of tiredness and fatigue
- Contributes to the regeneration of the reduced form of vitamin E
- Increases iron absorption
It is for the food manufacturer to decide which of these claims, if any, are relevant for specific products. And then comes the challenge of reformulating whilst delivering on as many as possible of the key criteria, in particular the shelf-life stability to ensure that the necessary level is present at the end of the product life.
Implementing a structured product reformulation programme is essential in achieving success in this arena. Consider the four main stages of the process, Design, Development, Implementation and Compliance, and consider the key inputs into each stage. It is always essential to consider what the consumer wants and whether the reformulation will add value to your product. This value may be in terms of meeting a consumer need, or in fact a permitted health claim that will allow your product to be re-positioned or marketed differently. However, reformulation may impact on the integrity and sensory profile of your product in such a way that may be detrimental so always consider the broader sensory acceptability of that reformulation. Leatherhead Food Research provides expert support services from Concept to Consumer and can ensure that your product meets all expectations.
For assistance in navigating this difficult area of reformulation please email: Dr Pretima Titoria, Consultant: Food Ingredients, Food Innovation, E: [email protected]
News
Keep apprised of the latest developments and news in the food and beverage industry.
Industry events
Leatherhead will be attending various Industry events throughout the year.